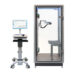
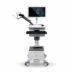
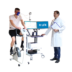
After intensive preparation, including extensive documentation as well as intensive and challenging tests for biocompatibility, cybersecurity and other relevant test criteria, GANSHORN received approval from the US Food and Drug Administration (FDA) for the commercialisation of the PowerCube+ series at the beginning of December 2024. This approval gives GANSHORN access to the world’s largest market in the field of pneumological diagnostics and means that the PowerCube Body+ and PowerCube Diffusion+ devices can now also be offered in the USA.
The PowerCube+ series from GANSHORN, which has been established for decades, stands for first-class quality and maximum precision in the diagnosis and monitoring of obstructive and restrictive lung diseases, as well as diffusion measurements and PFT tests. The focus is always on the patient and their health through continuous monitoring and ease of use.
All products in the PowerCube+ series are equipped with the innovative and calibration-free SharpFlow ultrasound sensor. This heart of our devices delivers fast, reliable and precise results that impress users and patients.
Marketing via SCHILLER Americas will begin immediately. The first sales opportunities have already been identified and there is great excitement about the successful approval, too.
We are also pleased to announce that all our PowerCube Body+ series devices have now also received MDR certification. This reflects our ongoing commitment to regulatory compliance and patient safety. To date, we have successfully brought the SpiroScout, SpiroDef and PowerCube+ series products in compliance with the requirements of EU MDR 2017/745.
GANSHORN would like to thank all those involved for their support during the authorisation process.